Enable HIPAA-safe performance marketing and analytics
Ours Privacy is a HIPAA-compliant CDP + privacy platform that unlocks optimized ads and analytics while making sure sensitive data never reaches non-compliant tools. Leverage best-in-class tools - Tag Management, GA4, Google Ads, Meta, and more - without sacrificing patient privacy.
Signed BAAs
Signed BAAs
SOC 2 Type II
SOC 2 Type II
USA-Hosted
USA-Hosted
No AI Data Processing
No AI Data Processing
As featured in…




















"We landed on Ours Privacy after exploring and trying some other solutions in the space. Their team's deep collaboration with our product team to help us set up, and even streamline, our data processes has been the best experience I've had with a software vendor."
"We landed on Ours Privacy after exploring and trying some other solutions in the space. Their team's deep collaboration with our product team to help us set up, and even streamline, our data processes has been the best experience I've had with a software vendor."

Wesley Braden
Wesley Braden
Senior Marketing Manager, Virta Health
Senior Marketing Manager, Virta Health
Why privacy-first marketing matters
Multi-million dollar settlements, shifting HHS guidance, and dozens of new state privacy laws make healthcare marketing complex and high risk.
Compliance
HIPAA and new state privacy laws create complex requirements for every healthcare marketer.
Compliance
HIPAA and new state privacy laws create complex requirements for every healthcare marketer.
Compliance
HIPAA and new state privacy laws create complex requirements for every healthcare marketer.
Risk
Hidden trackers and non-compliant tools have already cost healthcare companies over $100M in lawsuits.
Risk
Hidden trackers and non-compliant tools have already cost healthcare companies over $100M in lawsuits.
Risk
Hidden trackers and non-compliant tools have already cost healthcare companies over $100M in lawsuits.
Trust
Patients expect privacy protection and choose providers who respect it.
Trust
Patients expect privacy protection and choose providers who respect it.
Trust
Patients expect privacy protection and choose providers who respect it.
Improper pixel usage can expose sensitive patient data, including IP addresses and browsing behavior, to third parties, risking severe privacy violations and regulatory penalties.
Ours Privacy Follows HHS Guidance
“The regulated entity can choose to establish a BAA with another vendor, for example a Customer Data Platform vendor, that will enter into a BAA with the regulated entity to de-identify online tracking information that includes PHI and then subsequently disclose only de-identified information to tracking technology vendors that are unwilling to enter into a BAA.”
Ours Privacy Follows HHS Guidance
“The regulated entity can choose to establish a BAA with another vendor, for example a Customer Data Platform vendor, that will enter into a BAA with the regulated entity to de-identify online tracking information that includes PHI and then subsequently disclose only de-identified information to tracking technology vendors that are unwilling to enter into a BAA.”
Ours Privacy Follows HHS Guidance
“The regulated entity can choose to establish a BAA with another vendor, for example a Customer Data Platform vendor, that will enter into a BAA with the regulated entity to de-identify online tracking information that includes PHI and then subsequently disclose only de-identified information to tracking technology vendors that are unwilling to enter into a BAA.”
Features built for healthcare marketing
You shouldn't have to choose between growth and patient privacy. Patients deserve to be helped, and you deserve the tools to reach them the right way.




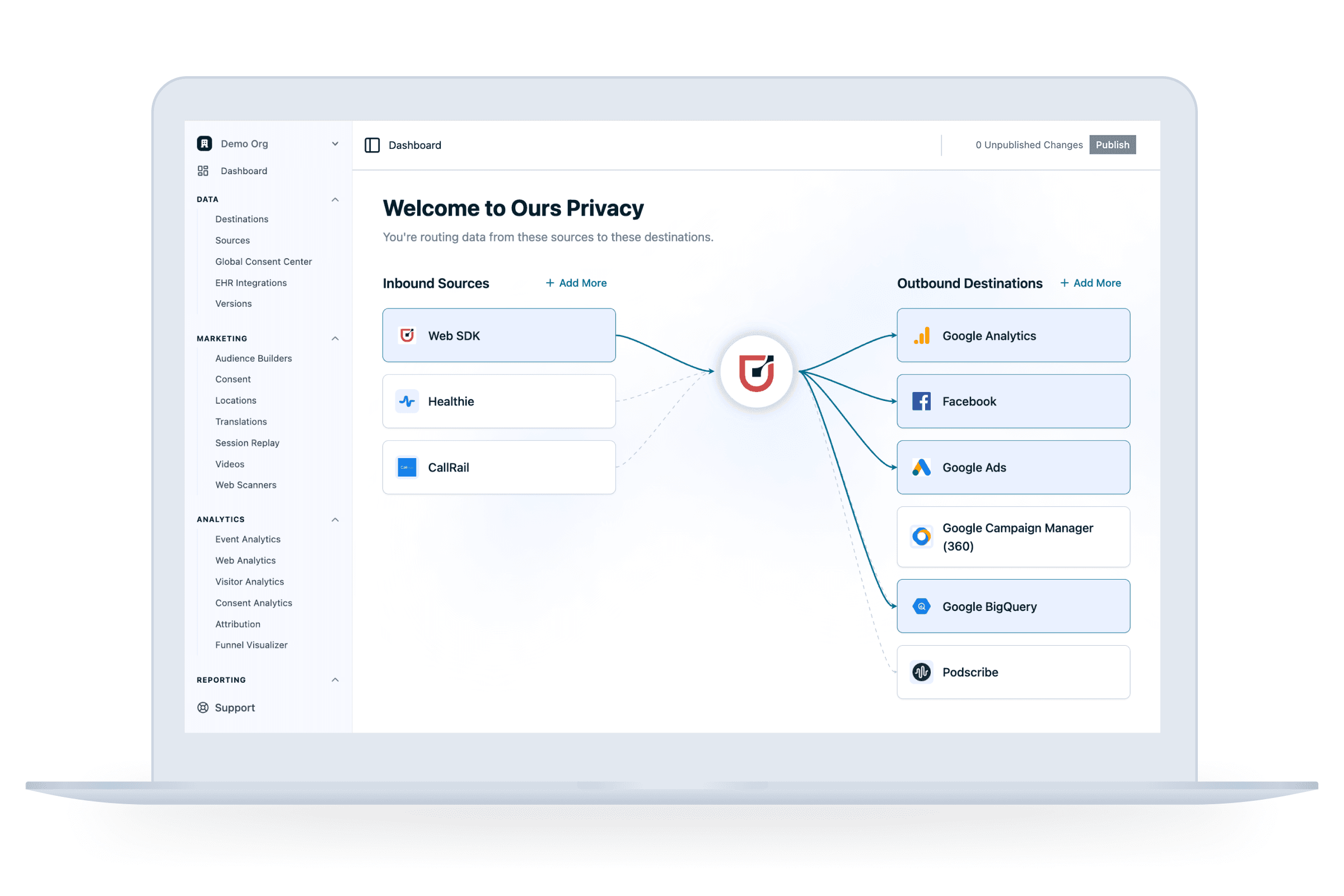
Get started quickly and securely
Continue to use all of the tools, events, and setups you know and love.
Signed BAAs
2-3 day implementations
Robust default settings
Secure infrastructure
Leverage advanced performance features
Access our industry leading optimization features to push your ROI even higher.
Toggle synthetic data
Advanced identity stitching
First-party custom domains
Multi-touch attribution


Trusted by high-growth startups to nationally-recognized health systems.
"Before Ours Privacy, our marketing was constantly scrutinized, and we struggled to confidently scale or optimize campaigns due to increasingly strict data restrictions.
Since implementing Ours Privacy, we’ve been able to satisfy all of our legal requirements, and scale our ad spend by 15% while maintaining the same acquisition costs. The platform gave us accurate, compliant, data and the confidence to be able to invest. The Ours Privacy team went above and beyond, even building custom integrations for us to make it all work.”
– Head of Growth, Leading Digital Health Company"The best thing about Ours Privacy is that it's invisible.
I've worked with lots of similar companies in the past and maintaining them took over my entire product and marketing roadmap at times. Ours Privacy takes basically zero time to upkeep. With everything going on with Meta, I feel so grateful that we found you and work with you. I would have had heart palpitations if not."
– CEO, Pediatric Behavioral Health Platform

"We cannot recommend them highly enough!
As a startup, our biggest stressor was the amount of time and team resources a platform would take to set up… Luckily, the process of setting up Ours Privacy was simple from start to finish. I was able to set up the tracking on my own, with no developer support. The Ours team put together a custom implementation plan that was clear, easy to follow, and customized to our needs. They even offered a call with us to check our set-up before we took it live. It was the most comprehensive and helpful onboarding check we have ever received from a software provider.
We are confident that our set-up aligns with best practices because of Ours’ platform and support.
– MyWellBeing

Having Ours Privacy is added peace of mind for us.
We wanted to make sure we dotted all our i's and crossed our t's when it came to compliance. This enables us to confidently scale over time and reach more women to provide high-quality care.”
— Jacqueline Parisi, Head of Marketing, Elektra

“Ours Privacy deeply understands the compliance and performance needs of healthcare marketers and I've loved the timeliness and level of support I've gotten from the team.”
– Josh Volkening, VP Growth, Allara
"Before Ours Privacy, our marketing was constantly scrutinized, and we struggled to confidently scale or optimize campaigns due to increasingly strict data restrictions.
Since implementing Ours Privacy, we’ve been able to satisfy all of our legal requirements, and scale our ad spend by 15% while maintaining the same acquisition costs. The platform gave us accurate, compliant, data and the confidence to be able to invest. The Ours Privacy team went above and beyond, even building custom integrations for us to make it all work.”
– Head of Growth, Leading Digital Health Company"The best thing about Ours Privacy is that it's invisible.
I've worked with lots of similar companies in the past and maintaining them took over my entire product and marketing roadmap at times. Ours Privacy takes basically zero time to upkeep. With everything going on with Meta, I feel so grateful that we found you and work with you. I would have had heart palpitations if not."
– CEO, Pediatric Behavioral Health Platform

"We cannot recommend them highly enough!
As a startup, our biggest stressor was the amount of time and team resources a platform would take to set up… Luckily, the process of setting up Ours Privacy was simple from start to finish. I was able to set up the tracking on my own, with no developer support. The Ours team put together a custom implementation plan that was clear, easy to follow, and customized to our needs. They even offered a call with us to check our set-up before we took it live. It was the most comprehensive and helpful onboarding check we have ever received from a software provider.
We are confident that our set-up aligns with best practices because of Ours’ platform and support.
– MyWellBeing

Having Ours Privacy is added peace of mind for us.
We wanted to make sure we dotted all our i's and crossed our t's when it came to compliance. This enables us to confidently scale over time and reach more women to provide high-quality care.”
— Jacqueline Parisi, Head of Marketing, Elektra

“Ours Privacy deeply understands the compliance and performance needs of healthcare marketers and I've loved the timeliness and level of support I've gotten from the team.”
– Josh Volkening, VP Growth, Allara
"Before Ours Privacy, our marketing was constantly scrutinized, and we struggled to confidently scale or optimize campaigns due to increasingly strict data restrictions.
Since implementing Ours Privacy, we’ve been able to satisfy all of our legal requirements, and scale our ad spend by 15% while maintaining the same acquisition costs. The platform gave us accurate, compliant, data and the confidence to be able to invest. The Ours Privacy team went above and beyond, even building custom integrations for us to make it all work.”
– Head of Growth, Leading Digital Health Company"The best thing about Ours Privacy is that it's invisible.
I've worked with lots of similar companies in the past and maintaining them took over my entire product and marketing roadmap at times. Ours Privacy takes basically zero time to upkeep. With everything going on with Meta, I feel so grateful that we found you and work with you. I would have had heart palpitations if not."
– CEO, Pediatric Behavioral Health Platform

"We cannot recommend them highly enough!
As a startup, our biggest stressor was the amount of time and team resources a platform would take to set up… Luckily, the process of setting up Ours Privacy was simple from start to finish. I was able to set up the tracking on my own, with no developer support. The Ours team put together a custom implementation plan that was clear, easy to follow, and customized to our needs. They even offered a call with us to check our set-up before we took it live. It was the most comprehensive and helpful onboarding check we have ever received from a software provider.
We are confident that our set-up aligns with best practices because of Ours’ platform and support.
– MyWellBeing

Having Ours Privacy is added peace of mind for us.
We wanted to make sure we dotted all our i's and crossed our t's when it came to compliance. This enables us to confidently scale over time and reach more women to provide high-quality care.”
— Jacqueline Parisi, Head of Marketing, Elektra

“Ours Privacy deeply understands the compliance and performance needs of healthcare marketers and I've loved the timeliness and level of support I've gotten from the team.”
– Josh Volkening, VP Growth, Allara

“Ours Privacy deeply understands the compliance and performance needs of healthcare marketers and I've loved the timeliness and level of support I've gotten from the team.”
– Josh Volkening, VP Growth, Allara
"Before Ours Privacy, our marketing was constantly scrutinized, and we struggled to confidently scale or optimize campaigns due to increasingly strict data restrictions.
Since implementing Ours Privacy, we’ve been able to satisfy all of our legal requirements, and scale our ad spend by 15% while maintaining the same acquisition costs. The platform gave us accurate, compliant data and the confidence to be able to invest. The Ours Privacy team went above and beyond, even building custom integrations for us to make it all work.”
– Head of Growth, Leading Digital Health Company"The best thing about Ours Privacy is that it's invisible.
I've worked with lots of similar companies in the past and maintaining them took over my entire product and marketing roadmap at times. Ours Privacy takes basically zero time to upkeep. With everything going on with Meta, I feel so grateful that we found you and work with you. I would have had heart palpitations if not."
– CEO, Pediatric Behavioral Health Platform

Having Ours Privacy is added peace of mind for us.
We wanted to make sure we dotted all our i's and crossed our t's when it came to compliance. This enables us to confidently scale over time and reach more women to provide high-quality care.”
— Jacqueline Parisi, Head of Marketing, Elektra

"We cannot recommend them highly enough!
As a startup, our biggest stressor was the amount of time and team resources a platform would take to set up… Luckily, the process of setting up Ours Privacy was simple from start to finish. I was able to set up the tracking on my own, with no developer support. The Ours team put together a custom implementation plan that was clear, easy to follow, and customized to our needs. They even offered a call with us to check our set-up before we took it live. It was the most comprehensive and helpful onboarding check we have ever received from a software provider.
We are confident that our set-up aligns with best practices because of Ours’ platform and support.
– MyWellBeing


Use Your Preferred Marketing Channels
Comprehensive ad and analytics integrations optimized with server-side connections, including GTM, GA4, Google Ads, Meta, and more. We add new integrations every week, just let us know what you need.
Use Your Preferred Marketing Channels
Comprehensive ad and analytics integrations optimized with server-side connections, including GTM, GA4, Google Ads, Meta, and more. We add new integrations every week, just let us know what you need.


Use Your Preferred Marketing Channels
Comprehensive ad and analytics integrations optimized with server-side connections, including GTM, GA4, Google Ads, Meta, and more. We add new integrations every week, just let us know what you need.

Start the conversation
Healthcare marketers tell us every day about the challenges of flying blind, navigating compliance, and dealing with ad restrictions. Talk with one of our experts to see if Ours Privacy is the right fit for your organization.

Start the conversation
Healthcare marketers tell us every day about the challenges of flying blind, navigating compliance, and dealing with ad restrictions. Talk with one of our experts to see if Ours Privacy is the right fit for your organization.

Start the conversation
Healthcare marketers tell us every day about the challenges of flying blind, navigating compliance, and dealing with ad restrictions. Talk with one of our experts to see if Ours Privacy is the right fit for your organization.
Learn more about our full-stack healthcare marketing engine
Simplify your marketing compliance stack with Ours Privacy, the modern healthcare privacy platform designed for healthcare marketers and trusted by engineering and compliance.
Launch in days or weeks, not months
Built by healthcare marketers for healthcare marketers, Ours Privacy makes it easy to get started quickly. With a modern, user-friendly interface, your marketing team can run campaigns autonomously — freeing up engineering to stay focused on the product.
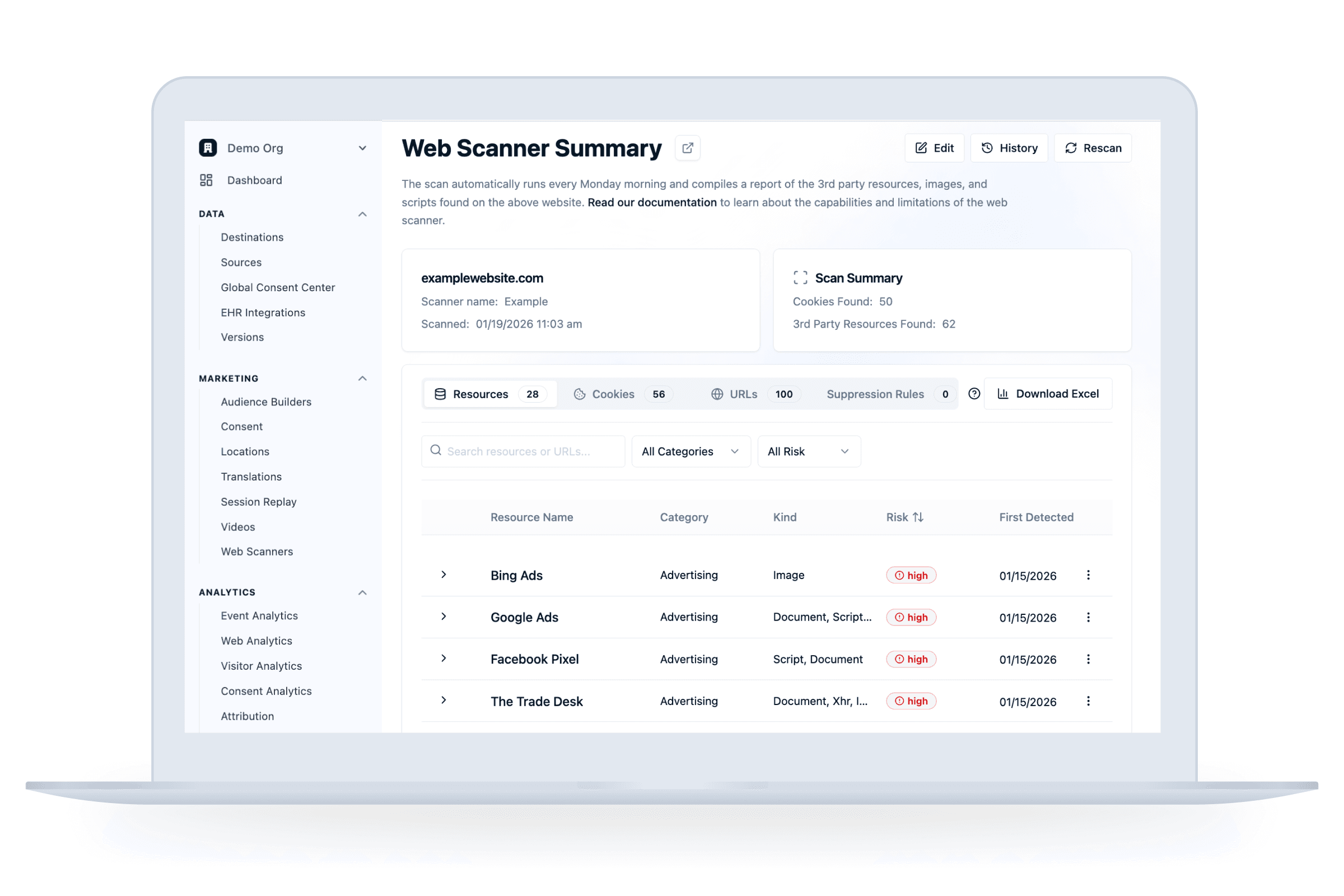
Step 1 - Install
Identify all third-parties on your site
—
Remove third-party pixels from your site
—
Set up the Ours Privacy tracking script in 5 min
Step 2 - Configure
Connect to Meta, Google Analytics, and over 50+ integrations
—
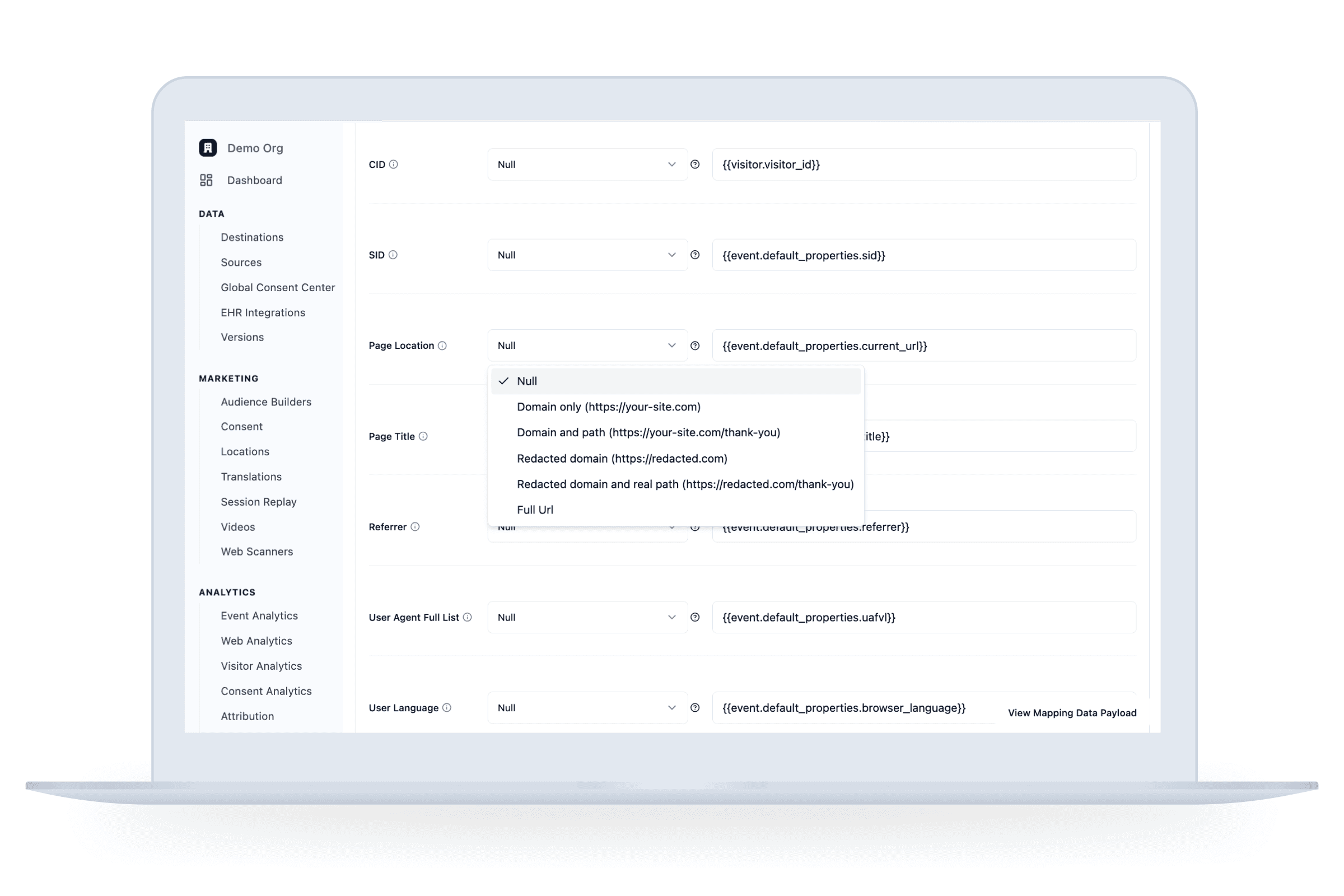
Redact, rename, hash, or otherwise anonymize each event and control what gets sent, where
Step 3 - Launch
Sleep soundly at night, knowing you are HIPAA-compliant
—
Clients often see ~ 2x reduction in CAC compared to flying blind
—
Quickly spin up new marketing channels on your own — no engineering resources required

"I was able to set up the tracking on my own, with no developer support.
As a startup, our biggest stressor was the amount of time and team resources a platform would take to set up… Luckily, the process of setting up Ours Privacy was simple from start to finish. The Ours team put together a custom implementation plan that was clear, easy to follow, and customized to our needs. It was the most comprehensive and helpful onboarding check we have ever received.
We are confident that our set-up aligns with best practices because of Ours’ platform and support. We cannot recommend them highly enough!
"I was able to set up the tracking on my own, with no developer support.
As a startup, our biggest stressor was the amount of time and team resources a platform would take to set up… Luckily, the process of setting up Ours Privacy was simple from start to finish. The Ours team put together a custom implementation plan that was clear, easy to follow, and customized to our needs. It was the most comprehensive and helpful onboarding check we have ever received.
We are confident that our set-up aligns with best practices because of Ours’ platform and support. We cannot recommend them highly enough!

"I was able to set up the tracking on my own, with no developer support.
As a startup, our biggest stressor was the amount of time and team resources a platform would take to set up… Luckily, the process of setting up Ours Privacy was simple from start to finish. The Ours team put together a custom implementation plan that was clear, easy to follow, and customized to our needs. It was the most comprehensive and helpful onboarding check we have ever received.
We are confident that our set-up aligns with best practices because of Ours’ platform and support. We cannot recommend them highly enough!
How much engineering support is needed to get started & maintain? Can marketing own this?
How much engineering support is needed to get started & maintain? Can marketing own this?
How much engineering support is needed to get started & maintain? Can marketing own this?
How much does Ours Privacy cost?
How much does Ours Privacy cost?
How much does Ours Privacy cost?
Can Ours Privacy help with Meta’s Health and Wellness restrictions?
Can Ours Privacy help with Meta’s Health and Wellness restrictions?
Can Ours Privacy help with Meta’s Health and Wellness restrictions?
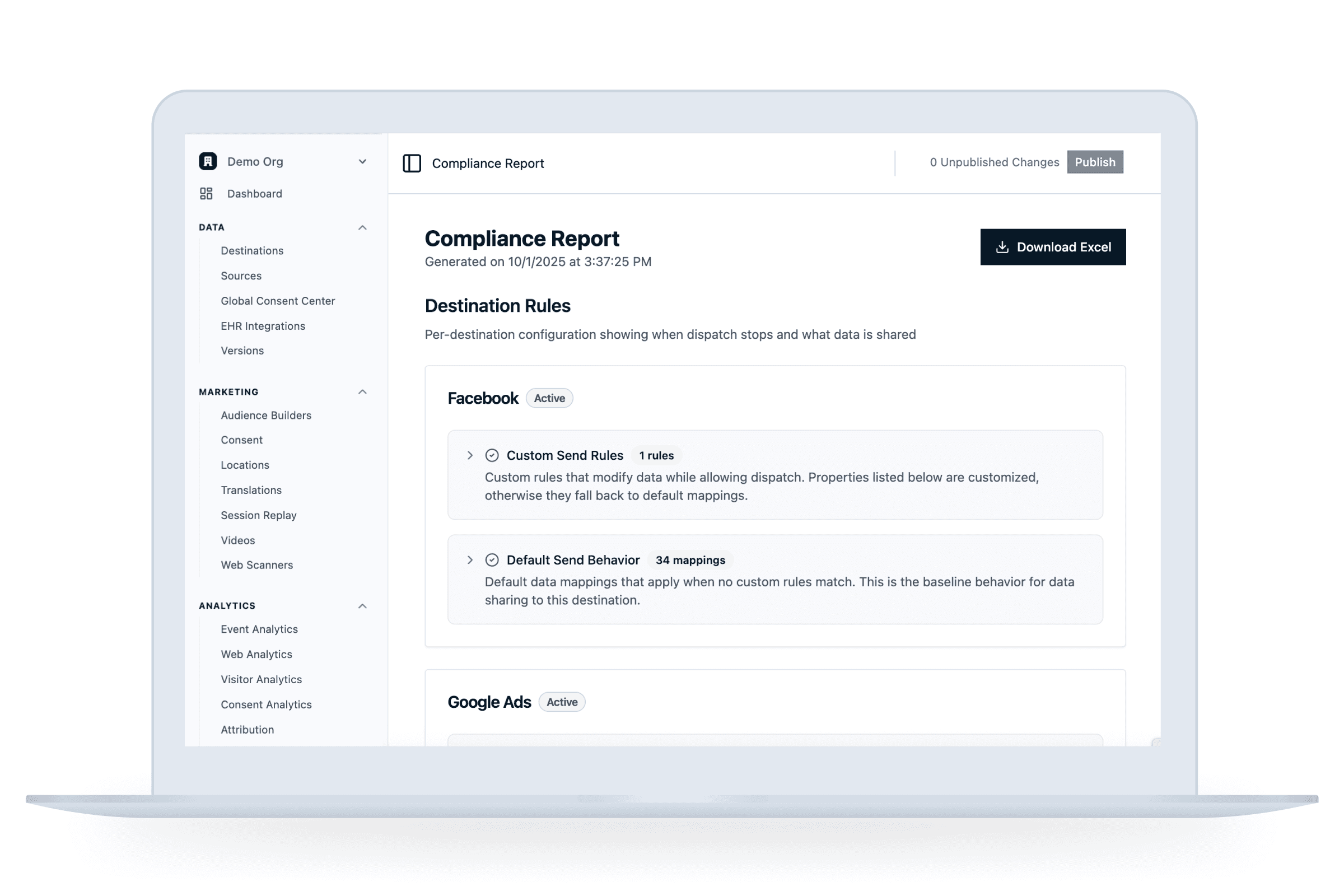
How does Ours Privacy prevent PHI from being sent to destinations and enable HIPAA-compliance?
How does Ours Privacy prevent PHI from being sent to destinations and enable HIPAA-compliance?
How does Ours Privacy prevent PHI from being sent to destinations and enable HIPAA-compliance?
What if I’m already using another tool, like Freshpaint?
What if I’m already using another tool, like Freshpaint?
What if I’m already using another tool, like Freshpaint?
Latest Insights on Privacy & Marketing
Dive into expert insights, product updates, and trends shaping the privacy forward analytics and healthcare marketing landscape.










